Thailand says it has asked Myanmar junta to reduce violence
European court ruling puts cautious Swiss in climate bind
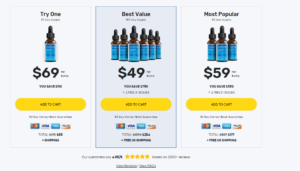
Keto BHB Salts Reviews (NTX KETO BHB GUMMIES) Metabolix Labs Keto ACV Gummies for Trim Tummy
Featured Posts
We must stop children getting addicted to online gaming
How Matches Lifestyle is investing in the designers of tomorrow
In a circular economy for fashion, clothes, shoes, and accessories are used more, are made from safe and renewable materials, and are made to be made again.
How Matches Lifestyle is investing in the designers of tomorrow
In a circular economy for fashion, clothes, shoes, and accessories are used more, are made from safe and renewable materials, and are made to be made again.
Fashion businesses are no longer a worthwhile investment
The scientists spoke to local people, who relied on the filthy streams and rivers for their water supply, and learned that waste from textile factories was contaminating the waterways.
Fashion businesses are no longer a worthwhile investment
The scientists spoke to local people, who relied on the filthy streams and rivers for their water supply, and learned that waste from textile factories was contaminating the waterways.
Florida Man Who Drove Into Protesters Gets 6 Years in Prison
Dozens of protesters sustained serious injuries from teargas, rubber bullets, stun grenades, used by police to disperse crowds during the Dark Holiday Protest.